hcl ir spectrum
We will use the Nicollet FT-IR to record the IR spectrum of HCl. The chloroniumyl cation HCl has been recently identified in space from Herschels spectra.

Hcl And Dcl Ir Absorption Spectra Piper Pchem Inspired Pedagogical Electronic Resourse
Using the Nicolet 6700 spectrometer the spectrum for HCl was analyzed.

. Simplest vibrating diatomic model is a harmonic oscillator described by. The chloroniumyl cation HCl has been recently identified in space from Herschels spectra. DIGITIZED BY NIST FROM HARD COPY FROM TWO SEGMENTS.
View the Full Spectrum for FREE. The chlorine isotope peaks are resolved to baseline. Infrared spectroscopy of HCL spectrum and animations of molecular motion.
There were two branches that were apparent in the result of the spectroscopy the R branch and the P branch that correspond to J 1 and J -1 respectively. The value for absorption is usually in cm -1. The infrared absorption spectrum of the HCl molecule is measured using a Fourier-transform infrared FTIR spectrometer.
Cm 2 566805 3 834698 4 1092311 5 1339655 PrecautionsNotes 1. D. GAS 200 mmHg N2 ADDED TOTAL PRESSURE 600 mmHg.
Spectra were collected on a Nicolet Nexus 670 FR-IR in a 10-cm gas cell at a pressure of 20 Torr. Before we start make a rough prediction. The KBr windows are used because glass absorbs strongly in the infrared region.
Does this have something to do with fact that peaks in IR spectroscopy represent areas of the spectrum where specific bond vibrations occur and therefore since HCl has a single bond it. Then find the corresponding values for absorption appearance and other attributes. Calbiochem EMD Chemicals Inc an Affiliate of Merck KGaA Darmstadt Germany.
To use an IR spectrum table first find the frequency or compound in the first column depending on which type of chart you are using. In the laboratory rather than prepare and analyze a fresh sample of HCl we will examine and manipulate a spectrum that has already been recorded and saved. Top References Notes Data compiled by.
HCl and DCl IR absorption spectra. In terms of wavenumbers the infrared region is located between 10-14000 cm-1. Note that not all frequencies have a related compound.
Use the infrared vibrational spectrum of HCl and DCl to obtain the following. Each table will have peak assignments and peak locations 37- 1one table for the H35Cl molecules and the other table for the 1H Cl molecules. GAS 200 mmHg DILUTED TO A TOTAL PRESSURE OF 600 mmHg WITH N2.
A joint analysis of extensive vis-UV spectroscopy emission data together with a few high resolution. Infrared spectrum of HCl 1 Create a table that associates peak frequencies in cm-1 with values of m for each isotopomer. The spectra from several isotopes of HCl are an-alyzed for common information about the molecular bond and for variations arising.
Suggest one reason why HCl has only one peak. Because Cl is much more electronegative than H HCl has a dipole moment. These windows are hydroscopic and thus the cell is kept in a dessicator.
The IR spectra of HCl solutions additionally show a broad continuum between the bending and the stretching peaks from 2000 to 3000 cm 1 and a broad peak at around 1200 cm 1 both of which. Hydrogen Chloride HCl HCl is a very simple example for demonstrating how molecules absorb radiation. The infrared region of the spectrum extends from 700 nm the long-wavelength end of the visible region to 1000 m the beginning of the microwave region.
PDF files of peak-labeled high resolution. Simplest rotating diatomic model is the rigid rotor or dumb-bell model which can be pictured as two masses joined by a rigid weightless rod and described by. 34 Chapter 6 Analysis of the Infrared Spectrum of HCl Band origins for the HCl infrared transitions.
The instrument used in this experiment an FTIR spectrometer can obtain IR spectra. Each peak differentiating between 35 Cl and 37 Cl is assigned an m value and then plotted with. HCl gas is placed in a cell with KBr windows.
View the Full Spectrum for FREE. When HCl vibrates this dipole moment think of it as an electric field oscillates. Because the reduced masses of these two isotopic forms of the HCl molecule are.
Attenuated Total Reflectance Infrared ATR-IR Spectrum. Infrared rovibronic spectroscopy of HCl. A joint analysis of extensive vis-UV spectroscopy emission data together with a few high-resolution and high-accuracy millimiter-wave data provided the necessary rest frequencies to support the astronomical identification.
The full spectrum can only be viewed using a FREE account.
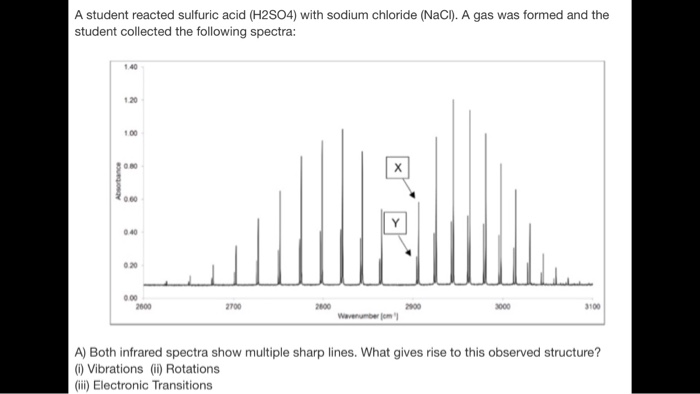
Below Is The Ftir Spectrum Of Hcl Chegg Com

Experiment 9 Rotational Vibrational Spectroscopy Introduction
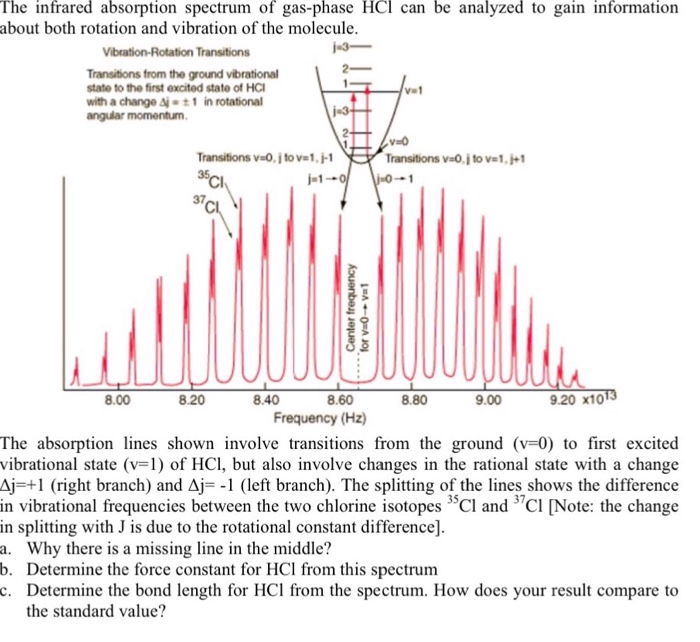
Solved The Infrared Absorption Spectrum Of Gas Phase Hcl Can Chegg Com

A Ir Spectrum Of The Deposit Formed By Co Condensing Hg Atoms And 2 Download Scientific Diagram
.jpg)
Analyzing The Gas Phase Spectrum Of Hydrogen Chloride With Ft Ir
Organic Nitrogen Compounds V Amine Salts
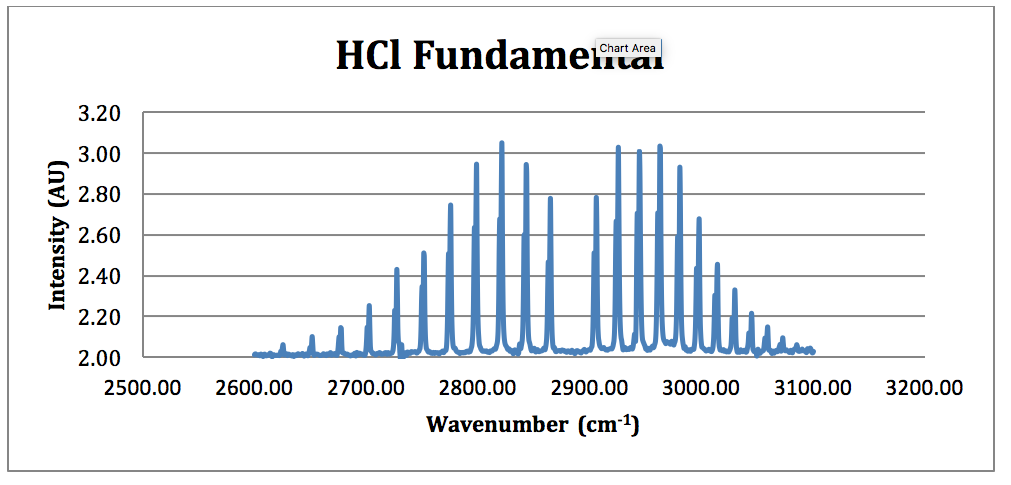
Spectroscopy And Molecular Structure Hci Dci

Ft Ir Spectrum Of Metformin Hcl Download Scientific Diagram

Ft Ir Spectra Of Pani Doped With Hcl Acid Download Scientific Diagram

Infrared Spectrum Ftir Of 4 Methoxyphencyclidine Hcl Download Scientific Diagram
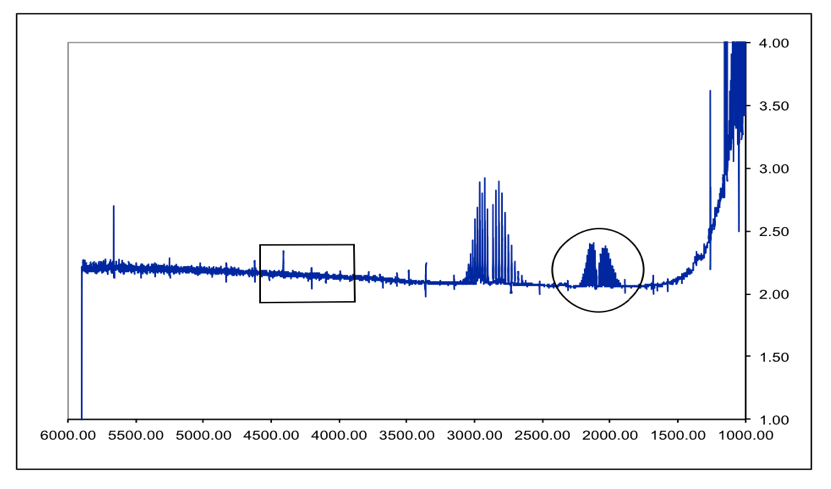
Spectroscopy And Molecular Structure Hci Dci

Infrared Spectrometric Rotational And Vibrational Analysis Of Hcl And Dcl Caroline Frank
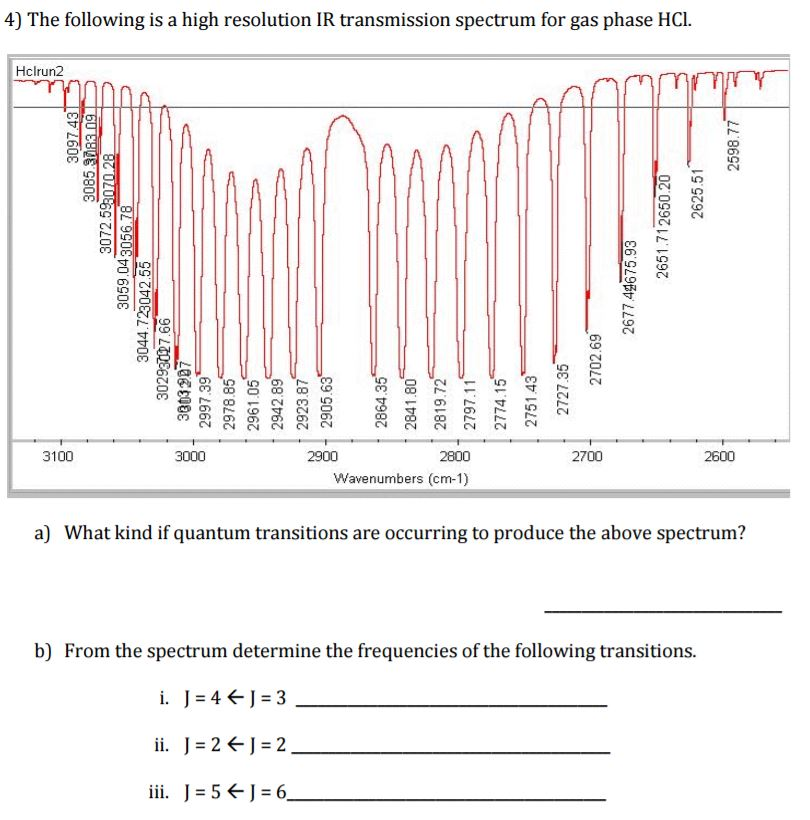
Solved The Following Is A High Resolution Ir Transmission Chegg Com

A Infrared Spectra Of Pure Water And 1 0 M Aqueous Solutions Of Download Scientific Diagram

0 Response to "hcl ir spectrum"
Post a Comment